Hello the internet!
I am Foaly the guy who made the carbonated molecular Tschunk spheres at the 33. Chaos Communication Congress in Hamburg. It got mentioned as things to take away from the 33c3 in the closing ceremony (you can switch the recording to english using the little star in the bottom right of the player). So to avoid explaining it over and over again, I thought it’s easier to write it down properly once. So here you go: have fun with it!
Tschunk
Tschunk is the hackers drink of choice. Making Tschunk is indeed as simple as it gets. It mainly consists of Mate and Rum. There is no fixed recipe. The ingredients are mostly the same, but the quantities may vary. Hackers usually mix it to their own taste. Here is a typical Tschunk recipe:
First things first though:

Tschunk Recipe
- some brown sugar
- half a lime
- squash the two ingredients in a glass
- add some (crushed) ice
- 4 seconds of your favorite Rum
- 10 seconds of your favorite Mate
- stir & enjoy
For more on Tschunk and pictures of a more elaborate recipe see tschunk.org.
Spherification
Now that you got the most important part, the Tschunk, lets look into making spheres out of it. The idea to turn liquids into little spheres with a thin gel membrane comes from Molecular Gastronomy. It was developed in 2003 at the el Bulli restaurant by Ferran Adria and his team. They originally used it to make something called Melon Caviar, which are tiny little gel spheres made from melon juice. The Tschunk spheres I made where a little bigger, around the size of a raspberry (the fruit not the computer). The funny thing about these spheres is that when you eat them you first have a firm but squishy gel sphere in your mouth (a bit like a soft, round gummy bear). As soon as bite on them though, they pop and release a splash of Tschunk in your mouth. I admit the feeling is a little alien like, but it’s a lot of fun! I was told it is a lot like the spheres in bubble tea, but since I have never tried bubble tea, I don’t know. I was always interested in this process, but when they talked about it in this years Nobel price special episode of my favorite podcast Methodisch Inkorrekt (german) and they concluded with the words: “… somebody should give this a try with alcohol …” I had to do it! :)
The following science part turned out longer than I expected. If you want to get straight to business, you can skip this block and go to the recipe directly.
Two ingredients are needed for spherification: Sodium Alginate and a Calcium salt, usually Calcium Lactate, which is what I used. Sodium alginate is made from brown algae and calcium lactate is manufactured from of lactic acid. Both have E numbers (E 401 and E 327 respectively) so they are considered safe for consumption. The working principle is described very well in the mentioned Methodisch Inkorrekt episode (chapter 12, 2:31:00).
When you break it down the science behind it is quiet easy to understand. The sodium alginate consists of really long molecule chains. If these chains come in contact with calcium ions they form bonds and the single molecule strands get all entangled into one big mess (gelification). A metaphor that is often used to describe this process is to think of spaghetti. A sodium alginate solution behaves like a pot of freshly cooked, still slightly wet spaghetti. There are no bonds between them, it is simple to pick them apart and they slip past each other easily. Now when sodium alginate is mixed with calcium ions it behaves more like a pot of cold and slightly dry spaghetti. They are all entangled into each other, they stick together and don’t easily slide past each other anymore and it is really difficult to pick them apart. If you apply enough force the entangled spaghettis/molecule chains will break, which is what you observe when you bite on a sphere.
So if you use this process to your advantage you can use it to form little spheres. There are two ways that worked well for me. For the first method I used a syringe to drip one liquid into the other. While falling a nice round drop forms. Important for the formation of a properly round drop is the fall time, so make sure you drop it from at least 50 cm. Immediately after falling into the other liquid the gelification starts. Since the liquid is a sphere when entering the other liquid, it stays spherical while gelifying from the outside. Unless you have a big syringe the spheres tend to be rather small (~5 mm diameter), which is nice to produce flavored caviar. I wanted bigger spheres tough, so what I did is to inject the liquid directly into the other using the syringe. Important for both of these methods is that the liquid that is dropped/injected is thicker than the liquid it is put into, so that it stays together and doesn’t mix before it can gelify. When the drop method is used this is also important for breaking the surface tension. More on that later, as this is quiet important!
As you might have noticed I was rather unspecific about what liquid to mix with what in the previous paragraph. That is because you can do it either way. The older version, which is also the one described in the Methodisch Inkorrekt episode, is called Basic Spherification. Here you mix the liquid you want to spherify with the sodium alginate and drop it into a bath of calcium lactate. Sadly this doesn’t work for liquids that already contain calcium (like milk) or that contain alcohol. Since we are interested in that we go for the second version. It is called Reverse Spherification. Here you mix the liquid you want to spherify with calcium lactate and submerge it into a bath of sodium alginate. Besides the possibility to spherify alcohol Reverse Spherification has a couple more advantages. The thickness of the walls is better controllable, the spheres last longer and most important it works with ALCOHOL !!1! If you are interested you can read more about the pros and cons of each method here.
How to make Tschunk spheres

Sodium Alginate bath
Now let’s start with the actual recipe for the reverse spherification. For that you first want to prepare the sodium alginate bath. The ratio is 0.5 g Sodium Alginate per 100 mL of distilled water. I used 400 mL of distilled water, so I added 2 g of Sodium Alginate. Since the Sodium Alginate powder doesn’t dissolve easily in water I used an immersion blender to mix the two properly. Since we mixed a lot of air into the bath while blending, you have to set it aside for at least 1 hour to let all the air bubbles disappear. If you have a vacuum pump (which I sadly don’t) you could speed up the process ;)
Important note: Use distilled water! For my first experiments I used regular tab water, but where I live there is apparently already quiet some calcium ions in the tab water. Those ions already react with the Sodium Alginate and turned the bath into a thick, honey-like consistency, which makes it impossible to properly drip something else into it. Using distilled water results in a bath that is only thicker that water by a tiny amount, which makes it much easier to work with. So to spare you some work, just use distilled water.
Prepare the Tschunk
As you probably guessed we need to add some calcium ions to our Tschunk now. First I shaked the Tschunk properly so the sugar and the lime would dissolve. Then I filtered it into a glass, so we only get the liquid and no leftover lime or sugar.
Next step is to mix the Calcium Lactate into the Tschunk. The ratio is 1 g of Calcium Lactate per 100 mL of Tschunk. Since the Calcium Lactate powder also doesn’t dissolve in water to easy by itself, I used the immersion blender again. Don’t worry about the mixture foaming up and bubbling while you blend it. I suspect the Calcium Lactate having a high surface area, because this also happens with non-carbonated liquids (I’ve tried apple juice before). I don’t know what exactly causes it, but it’s normal.
To make it easier to form nice round drops while dripping the Tschunk into the Alginate bath I thickened the Tschunk/Calcium lactate mixture a little bit. This has also the nice effect that it makes the spheres slightly more durable. In molecular cuisine this is usually done by adding some Xanthan to the liquid containing the calcium (Tschunk). Since I didn’t have Xanthan available, I used guar gum (deutsch: Guarkernmehl) which I had laying around at home. I added a total 1 g of guar gum to the 400 mL of Tschunk I had prepared. You can uses whatever thickener is available to you. The important part is that the Tschunk mixture is thicker than the Alginate bath.

Making process
Now that you suffered though all the science and all the preparation, comes the exciting part of actually making the spheres! So we got our Tschunk calcium lactate mixture and our sodium alginate bath. Last thing we need is a water bath to rinse the spheres afterwards. Like I said I used a syringe to slowly inject some of the Tschunk mix into the alginate bath.

I left the spheres in the alginate bath for sometime between 30-60 seconds. Afterwards I scooped them out with a spoon which had some holes in it (a sieve also works very well) and put them into the water bath. That stops the reaction. This is another advantage of reverse spherification. The longer you leave the spheres in the alginate bath, the thicker the wall of the membrane will become. As soon as you take the spheres out of the alginate bath and rinse them in a water bath the gelification stops. This is not true basic spherification, where the reaction keeps on going, even after the spheres are put into a water bath, which leads eventually to a completely gelified sphere.

On the picture above you can see my “production line”. In the bowl on the left is the Tschunk mixed with calcium lactate and guar gum. The container in the middle is the sodium alginate bath. You can see three bigger spheres currently “cooking”. The rectangular container on the right is the water bath.
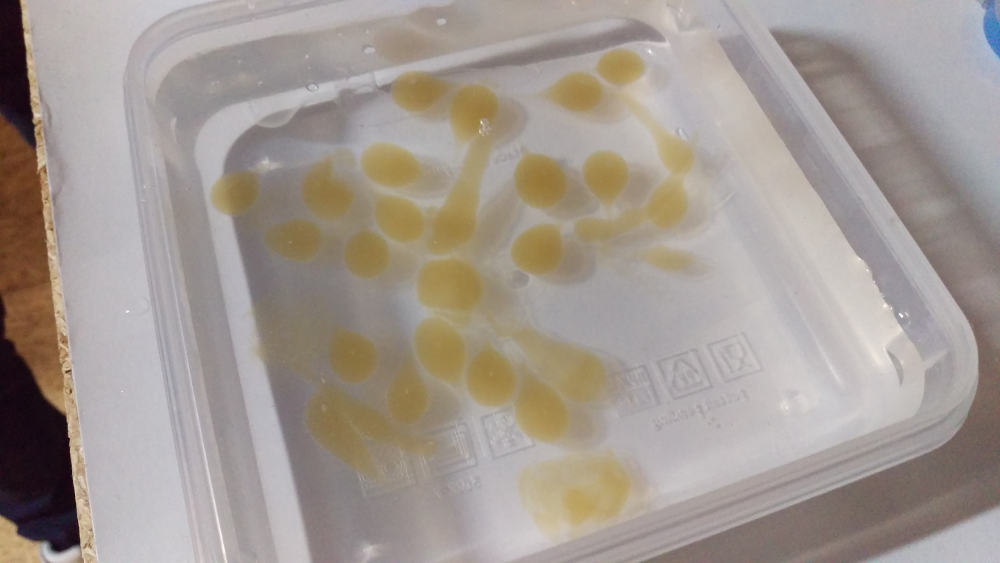
And there you have it. That’s how you make molecular Tschunk spheres.
Carbonated advanced version
One thing I really like about mate and Tschunk is the ridiculous amount of carbon dioxide in it. Of course all that get lost in the process of blending the Tschunk, but there is a way to put the fizziness back into the molecular Tschunk spheres.
In order to do that a cream syphon is needed. A cream syphon is basically a pressure vessel with a release valve, that you can screw little capsules on top to pressurize the container. Now how do we get the gas into the spheres? Biology to the rescue! The spheres consists out of the liquid in the middle and a gel membrane surrounding it. Membranes allow the process of osmosis to happen. Osmosis allows solvent molecules to travel through membranes. This is also important for storing the Tschunk spheres. If you store the spheres in water the Tschunk will dissipate through the membrane and the liquid inside the spheres will water down. So if you want to store the spheres for some time make sure to put them in Tschunk. That will keep them from watering down.
Back to carbonation. To carbonate the spheres put some for your remaining Tschunk in the cream syphon. Then add the spheres into the syphon. Close the syphon and charge it with a CO₂ capsule. Then carefully give the syphon a good shake and put it in the freezer. I have a 0.5 liter syphon and for my first attempt (which was at 33c3) I used 1 1/2 capsules (put in one, vent half, put in another). I was a bit scared to overcharge it, because the manual says not to do so and pressure vessels are to be treated with respect. I have since talked to some people at congress who have some more experience with syphons and learned that the syphons have all kinds of safety releases, so it is fine to use 2 capsules. Be careful though and do so at your own risk. You will be totally fine with using one capsule, but the process will take longer and the spheres will obviously be less carbonated. There is lots of room for experimentation here.
Important: Make sure you actually use CO₂ (carbon dioxide) capsules (usually labeled Soda Capsules). For whipping cream N₂O (nitrous oxide) capsules are used (usually labeled Cream Chargers). Those will obviously not work.
According to other recipes 30 minutes should be enough time for the CO₂ to dissolve into the Tschunk and diffuse through the membrane into the sphere. Once you take the syphon out of the freezer, you have to slowly vent the gas so that the spheres don’t rupture. After that you can open the syphon et voilà! You have successfully made molecular spheres containing carbonated Tschunk!
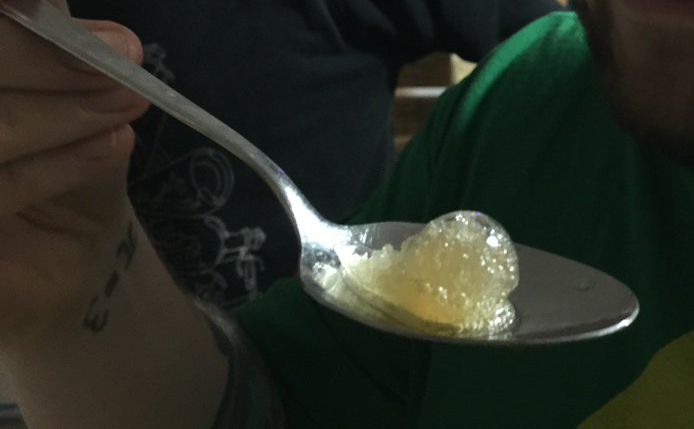
By accident I left the syphon in the freezer for about 90 minutes. I haven’t measured the exact temperature, but the freezer at the food hacking base was quiet strong. After I took the syphon out of the freezer and opened it, I saw that the entire Tschunk in it had turned to Tschunk slush. At first I was worried that it might have not worked, but than I realized that ALL the Tschunk had turned to slush. Even the Tschunk inside the spheres. So I accidentally ended up with molecular spheres containing carbonated Tschunk slush! Which was just plain awesome!
Since at congress was the first time I actually tried to make the carbonated spheres, I don’t know if the spheres will turn to slush every time if you leave them in the freezer for a longer time. Again lots of room for further experimentation and improvement!
Copy Cats Welcome!
If you feel like making your own molecular spheres now, please do so! It is indeed a lot of fun! Also don’t forget to tweet me some pictures of your results! If you don’t drink alcohol this whole process works of course with pretty much every possible liquid. Like I mentioned I haven’t tried a lot of different things, but for the first experiments I used apple juice and that worked very well.
If you have further questions or improvements please also feel free to message me on Twitter. If you find mistakes in this text or you have suggestion on how to improve it, please message me on GitHub or send a pull request.
Thanks
I would like to say thanks to all the people who helped me with this stupid idea. Especially Chris, who helped me actually making the spheres at congress, the awesome people at the Food Hacking Base for providing the infrastructure, Reinhard and Nicolas from Methodisch Inkorrekt for the idea and for featuring the result in the closing ceremony \o/ and my room mates for suffering through the first iterations. Thanks again to everybody!
Small scale hack
There is one more funny little anecdote I have to tell you about, that happened to me at the congress while making the spheres. In order to properly measure the ingredients I used a fine scale with the a resolution of 0.1 grams. I’ve had it for a while and when I used it at home it always worked reliably. When I turned it on at Congress though it read some error I had never seen before. Getting a little anxious, because I needed the scale in order to make the spheres, I started pushing ALL the buttons, like a true professional. Funny enough I got to the number read out and the numbers where fluctuation like crazy. They would happily jump around. Sometimes it was -2.5, sometimes 0.7, 0.1, 5.4, … You get the idea. The scale was basically useless.
So then somebody observing my misery suggested: “Just put the thing in the microwave.” And what do you know… even with the door open the scale went back to working perfectly (except for the batteries that died shortly afterwards, but luckily I knew how to fix that). Later when I told the physicists from Methodisch Inkorrekt about it Reinhard was able the explain to me why it worked. Apparently electric fine scales work with a little magnet that moves up and down in a coil. By measuring the current in the coil the weight pushing down can be calculated. Now knowing that some of you might have already guessed what happened… :) Since at Congress ALL the spectrum is used EVERYWHERE all the time there was so much radio waves in the air, that it induced a current into the coil of the scale. That of course completely messed up the reading. The fix by putting it into the microwave works, because microwave ovens are Faraday cages (you really want to keep the microwaves inside the box!). So by putting the scale inside the microwave it was shielded from all the radio waves outside.
I just wanted to include this to give you yet another example of the awesomeness, helpfulness and friendliness of the people at Congress! It showed me again that with some ingenuity, clever thinking and help from other people you can solve problems that seem unsolvable, overwhelming and frustrating to you.
And I guess that is what Congress is all about! :)
Links
Here are some links that I used for research:
The site http://www.molecularrecipes.com/ has a shit ton of recipes from molecular gastronomy. They try to sell you their kits and advertising them all over the site, but the amount of information is just amazing. Besides the recipes, they have online courses describing technics, informational sites and Tips & Tricks (those where especially helpful!).
- Overview page about spherification: http://www.molecularrecipes.com/spherification-class/
- Spherification history (for the interested): http://www.molecularrecipes.com/spherification-class/el-bulli-spherification-history/
- Excellent description of Reverse Spherification (this was my main source I’d say): http://www.molecularrecipes.com/spherification-class/reverse-spherification/
- Tips & Tricks about spherification: http://www.molecularrecipes.com/spherification-class/10-tips-create-perfect-sphere/
- Carbonated Mojito Spheres (a similar recipe): http://www.molecularrecipes.com/spherification-1/carbonated-mojito-spheres/